
At the beginning of May, the IPO of the first unicorn drug Aokang Deke of A shares appeared on the first day of the listing, soaring 44%, becoming the market value of 32.4 billion yuan, China's first, the world's eleventh small molecule pharmaceutical research and development giant. In the latter half of the year, "Yuanming Kangde has sealed 14 daily limit boards, with a market value of 111.9 billion yuan, ranking among the market value of the pharmaceutical sector.
Coincidentally, the first unlisted company, Ascletis Biotech, was listed on the Hong Kong Stock Exchange. This is the first section of the biology department that was born after the Hong Kong Stock Exchange opened the new listing system. It can be said that biopharm has ushered in a window period. If the former Huada gene has brought the distance between the public and the advanced modern medical means, genetic testing and diagnosis have entered the public life; and the big health industry has produced a number of digital medical and private hospitals such as the Lilac Garden and the United States. It is seen that China will also be regarded as a very stable industry for entrepreneurs, investors and workers who are gradually entering Japan into an aging society.
Then the contract research and development service (CRO) and contract manufacturing service (CMO), which is hailed as “Foxconn in the pharmaceutical industry†and “Huawei in the pharmaceutical industryâ€, demonstrates the great possibility of China overtaking in the biomedical industry. . Prior to this, people's memory of medical care mostly stayed in the top three hospitals in the big cities, rural medical care with great gaps in urban medical conditions, chain pharmacies, many generic drugs, and a variety of cosmetic hospitals and professional hospitals. Now, biomedicine shows us a new economy in a fashionable way.
Jiao Zhen, president of CDH Investment, said at an industry forum that “the most promising future in China is the health sector. The most promising areas of health are the pharmaceutical industry. The most promising in the pharmaceutical field is biopharmaceuticals.â€
In the United States, the biopharmaceutical industry is a very growth industry. As the world leader, the US biomedical industry can measure the current and prospects of China's biomedical industry from many dimensions.
We examine the biopharmaceutical industry in the US and China from the perspective of R&D expenses, market capitalization and sales revenue of listed companies.
Sino-US biomedical industry differences
R
The R&D investment in the biopharmaceutical industry is huge, usually accounting for 10%-20% of revenue, and the development cycle is also very long. According to the Tufts Center, the statistics on drug development research conducted in 2014 will take 10 to 15 years to bring a new therapy to market, with an investment of up to $2.5 billion.
The United States is a complete market economy, and the research and development costs of biomedicine are mainly invested by private companies. In the United States, between 2007 and 2012, investment in private pharmaceutical companies fell by $13 billion from $83 billion to $70 billion in 2012. Although the US government's investment has been stable for the same period, it is 480-490 billion US dollars. In total, in 2012, the two totals were 118-190 billion. Second, the investment of Chinese companies increased by 4.8 billion U.S. dollars from 1.5 billion U.S. dollars to 6.3 billion U.S. dollars in 2012, and Chinese government investment also increased rapidly, increasing by 1.4 billion U.S. dollars and reaching 2 billion U.S. dollars in 2012. . The total investment in 2012 was $8.3 billion.
In fact, a large part of Chinese companies' investment comes from the establishment of R&D centers in Europe and the United States because of subsidy policies, labor costs and a huge market. At present, the top 20 pharmaceutical companies in the world basically have R&D centers or branch offices in China. In terms of weight, the United States spent more than half of the world's biomedical research and development expenditure in 2007, reaching 51.2%; by 2012, it fell to 45.4%. The proportion of China's biomedical research and development expenditures in the world has soared from 1.7% in 2007 to 4.9% in 2012.
Therefore, as published in the first issue of the New England Journal of Medicine (NEJM) in 2014, the global biomedical research and development expenditures, Asia has begun to show glory. Since 2013, the US R&D expenses have remained at the original level, which is the level of 100 billion US dollars.
In the United States, another source is the National Institutes of Health (NIH). By the end of 2018, the hundreds of billions of dollars invested by the NIH in the past seven years directly or indirectly contributed to the birth of 210 new drugs. But the Trump administration plans to reduce the NIH budget. It can be seen that this kind of US investment in research and development is gradually falling.
In recent years, China's research and development efforts have been further strengthened. According to Wind Data, from 2015 to 2017, the research and development expenses of 63 listed companies in Shenyin Wanguo Biological Products Plus Pharmaceuticals were approximately 20.4 billion, 24.4 billion and 30.6 billion, respectively, steadily increasing. In the latest financial report disclosed in 2018, 81 pharmaceutical companies invested more than 100 million yuan in research and development in 2017. Among them, Hengrui Medicine and Fosun Pharma (Hong Kong stocks 02196) spent more than one billion yuan in research and development in 2017. China's pharmaceutical research and development efforts continue to increase.
Listed company
The most concentrated areas of the US biopharmaceutical industry cluster are Boston and San Francisco. Biopharmaceutical companies are growing well and the stock price volatility is also high. The share price of biopharmaceutical company HTG Molecular Diagnostics has soared 450% in two days. According to the Sina Financial US stock market, there are 67 pharmaceutical listed companies in the United States. According to Shenyin Wanguo, China's A-share pharmaceutical bio-sector has 275 listed companies, including 36 bio-products.
According to the US biopharmaceutical journal Genetic Engineering & Biotechnology News, the “Top 10 Global Market Value Pharmaceutical Companies in 2017†list has a market capitalization of US$1.722 trillion.
Table 1: Top 10 pharmaceutical companies in the 2017 GEN rankings
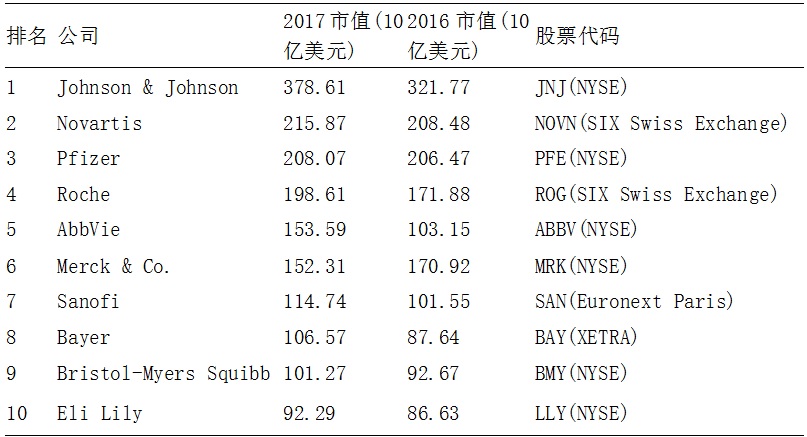
As can be seen from the above list, six of the top ten companies are listed companies of the US NYSE. According to Forbes, among the top 2000 global listed companies in 2017, 47 were pharmaceutical companies, and the United States and Japan were the most selected companies, all of which were 11 companies. Five companies in China entered the list, namely Sinopharm. Kangmei Pharmaceutical, Hengrui Medicine, Yunnan Baiyao, Fosun Pharma. It can be seen that China's pharmaceutical companies entering the list are still traditional pharmaceutical companies.
Sales revenue
Overall, the United States accounts for about half of the world's companies and half of the patents in biomedicine. Its sales of biomedical products account for more than 50% of the global biomedical products market. According to authoritative market research organizations, in 2008, US prescription drug sales increased by only 1.3% to $291 billion; prescription drug sales increased by only 0.9%.
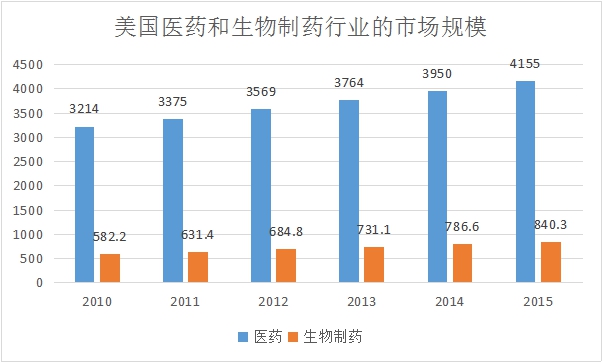
From 2010 to 2015, the US pharmaceutical market grew from $321.4 billion to $415.5 billion, while the biopharmaceutical industry's market size was $58.22 billion to $84.03 billion.
According to Frost & Sullivan, the market size of China's biopharmaceuticals increased from 62.7 billion yuan in 2012 to 152.7 billion yuan in 2016, with a compound annual growth rate of 24.9%. It is expected to grow at a compound annual growth rate of 16.4% from 2016 to 2021 and reach a market scale of 326.9 billion yuan in 2021, which will bring huge opportunities for Chinese biopharmaceutical participants.
Looking at the biomedical industry in China and the United States, the biomedical industry, which has received much attention in the United States, has also attracted great attention from China. From the above comparison, we can see that the bio-pharmaceutical industry, compared with the United States, China is probably only 10%-20% of the US.
However, with the rise of China, after the last round of urbanization, China's economic base can already consider the development of innovative high-tech industries. 5G, artificial intelligence, bio-pharmaceuticals, new energy, with the reshuffle of various fields with the upgrade of technology, does China's biomedicine have the conditions for cornering overtaking?
If we follow the same speed as the United States, it is difficult for us to make a leap. Where does the acceleration come from?
China's opportunities
Large sample data based on large population
With the exponential growth of big data, new technologies mature. Biomedicine combined with artificial intelligence methods will greatly improve R&D efficiency, but the big data fed to machine learning is the key to a decisive battle. Biopharmaceuticals have very high requirements on the amount of data in the research and development phase and the drug use phase.
For US pharmaceutical companies, obtaining high quality samples requires extremely high collection costs. For example, it may take five years to accumulate 12 million quality cases. China has a natural advantage in this regard. Getting the same sample may take only 5 days for a digital healthcare facility, and there are no problems with different sources of data. The large sample brought by the huge population will bring great advantages to the development of new drugs in China and biopharmaceuticals.
The future capital market will open a green light
The capital market is an excellent source of living water and an important intermediary to support economic transformation, new economic forms, and resource allocation. According to a recent speech by the chief economist of the Hong Kong Stock Exchange, Ba Shusong, the HKEx’s request for biopharmaceutical listing is “there is a new drug through the first phase of clinical, at least one complex investor, 1.5 billion in volume.†The Hong Kong capital market itself accommodates a lot of stocks with very small market capitalization. Therefore, the Hong Kong market provides great convenience for biopharmaceutical companies to seek listing and obtain capital support.
In the capital market of mainland China, the access conditions for listing have risen from 30 million in three years to 100 million. In 2018, the IPO meeting rate is less than half of 2017. But even so, in the next 10 years, 5G, biopharmaceuticals, artificial intelligence, etc. are listed as priority industries for listing. Recent listings have supported this trend. “No equity, no wealth†After the concept of the bio-industry has been so popular for a long time, the green light of the capital market has provided an excellent environment for the company’s financial value and its development.
New phase of consumption upgrade
With exports, consumption, and investment in the troika, the post-WTO exports drive GDP for a long period of time, while investment in fixed assets pushes up real estate prices. After the two horses are on the scene, the fast-growing middle class, the new generation who have been pursued by higher education for their quality of life will soon become the main force of consumption, which is also popular among economic policy makers.
In fact, it is obvious that various specialized hospitals have spread all over the first-line and new-tier cities and have occupied the market because of their more humane services. With the spurt of biopharmaceuticals, people are full of imaginations about curing severe diseases. It is expected that there will be more and more people willing to pay for new special effects.
Top-level design
From the policy design, the “Thirteenth Five-Year Plan†for biomedicine has been basically formulated, and biotechnology drugs are the innovative drug categories that are key to the development of China's biomedical industry during the “13th Five-Year Plan†period. According to the plan, “by 2020, it is necessary to promote a large number of biopharmaceutical enterprises to achieve drug quality standards and systems in line with international standards. At least 100 pharmaceutical preparation companies have obtained certifications from the developed countries such as the United States, Europe and Japan, and WHO, and achieved drug exports; In accordance with international drug standards, develop and promote 10 to 20 chemical drugs and high-end preparations, 3 to 5 new Chinese medicines, and 3 to 5 new biotech drugs to complete drug registration in developed countries in Europe and America, and accelerate their entry into the international market."
The scientific research layout shows the state's investment in biopharmaceuticals.
According to the plan, “By 2020, we will promote 3 to 5 well-established colleges and universities to form a large-scale pharmaceutical R&D base with high-tech and R&D capabilities, and establish a national-level transformation science center and collaborative innovation center to break through. 10 to 20 major core key technologies, initially establishing a national drug innovation system and innovation team, and bringing China's pharmaceutical industry system to the international advanced level, and promoting some key pharmaceutical companies to gain a foothold in the international market, striving to achieve an average international sales revenue of over 100 The level of 100 million yuan." It can be seen that from the state to the provinces, the support for medical mathematics is increasing.
The huge market and consumption power, the strong support of the capital market, and the huge data sample of artificial intelligence, these elements of European and American pharmaceutical companies are highly valued, and China's biopharmaceuticals are all available. Moreover, biopharmaceuticals have risen to a national strategy, and we have reason to believe that in the future China will achieve a cornering overtaking for the birth of more biopharmaceutical unicorns. (Source: Titanium Media)
GMP Certificated Immune Globulin Injection Supplier in China
Hepatitis B Immunoglobulin,Hep B Immunoglobulin,Hepatitis B Immunoglobulin Vaccine,Hepatitis Immune Globulin
FOSHAN PHARMA CO., LTD. , https://www.foshanpharma.com