Rapid expansion of human T cells using a breathable bag in the New BrunswickTM S41i CO2 thermostatic shaker
Amanda Suttle, Stacey Willard, Ma Sha
Enfield, Connecticut, United States Contact: sha.
Summary
Advances in cell therapy in recent years have greatly increased the demand for T cell amplification technology. T cells must be able to rapidly expand to achieve high cell densities while maintaining high survival and T cell properties. The stirred tank bioreactor is one of the best cell culture tools for large scale expansion. However, cell culture in a bioreactor requires the inoculation of a large number of cells. We have developed a method for inoculating a full-volume T cell using a vented bag for a benchtop bioreactor.
We used a vented bag placed in a New Brunswick S41i CO2 shaker for suspension T cell culture and tested cell culture bags from different manufacturers with maximal perfusions ranging from 120 mL to 5 L. In addition, we also tested different oscillation velocities to determine the optimal conditions for T cell expansion. Since T cells cannot grow on a fixed platform, proper oscillation is required. We also explored a more traditional method of suspension culture using an oscillating shake flask. The results showed that the use of breathable bags was more successful, with more than 10 times the amount of amplification in less than 4 days and only 2-3 times in shake flasks. In summary, we have established a method for large-scale preparation of cultured T cells in a stirred tank bioreactor using a venting bag and a CO2 constant temperature shaker.
Introduction
In the past few years, many research and development laboratories have been investigating conditions that enable the growth of large numbers of T cells to support the growth in demand for high quality T cells in cell therapy applications. The primary goal of T cell culture in blood bank laboratories has been to maintain cell populations for subsequent infusion. The technology is not yet able to meet the level required for R&D and commercial use. The use of bioreactor culture to amplify cells has been well documented, but has not been fully optimized for lymphocytes. In fact, T cells are sensitive and fragile, and previous experiments have shown that it is difficult to inoculate enough cells for desktop bioreactors.
Other suspended mammalian cells, including sensitive cells such as stem cells, can be successfully cultured in shake flasks using a CO2 controlled constant temperature shaker as previously indicated [1]. However, our preliminary study on the culture of primary human T cells using the shake flask method has not been successful. Therefore, we have developed a new method for cultivating these T cells by oscillating venting bags in the New Brunswick S41i CO2 constant temperature shaker (Abbend, S41I1200100). This method significantly optimizes T cell culture and yields rapid and efficient initial amplification conditions that enable the production of sufficient T cells for inoculation into a benchtop bioreactor. For example, in a benchtop study, we used a BioBLU® 3c disposable canister (Ebend, 1386000300) set to a maximum working volume (3.75 L) and a target cell seeding density of 0.5 x 106 cells/mL. A total of 1.88 x 108 cells are required for reactor inoculation. The study also established a method for cryopreservation of T cells, which is crucial for this study. The same batch of cells can be used for multiple optimization experiments by establishing a cryopreservation library.
Materials and Method
This study used a New Brunswick S41i Co2 constant temperature shaker for T cell expansion. A complete list of equipment and reagents is shown in Table 1.
Table 1: List of equipment, consumables and reagents used in this study
   |
Media Formulation and Constant Temperature Shaker Setup <br> Complete media formulation for this study included Lonza's X-VIVO 10 basal medium (without gentamicin and phenol red), plus 5% heat inactivated human serum 2 mM glutamine, 2 mM antibacterial-antimycotic, and 20 μg/mL IL-2.
In all studies, the S41i Co2 constant temperature shaker was set to a 5% CO2 level. The T cell culture grown in the suspension flask was placed on a stand. T cell cultures grown in Evolve bags were placed on an oscillating platform and taped to the platform to secure the sides of the bag (Figure 1). After holding the bag, set the shaker to the default 70 rpm oscillation speed (unless otherwise stated). These settings and media formulations were also used in the remainder of the study. As shown in Figure 1, the S41i Co2 constant temperature shaker can be used in a variety of different T cell culture methods.
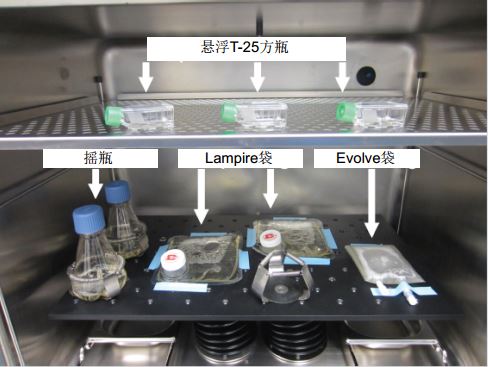
Figure 1: The S41i constant temperature shaker supports both shake flasks, suspended T-25 square vials and cell culture bags for T cell culture.
Optimizing T-cell Thawing and Initial Culture in Evolve Breathable Bags <br> We used two different methods for freezing a bottle of primary human peripheral blood T-cells purchased from STEMCELL Technologies. Both methods were used. The ThawSTAR CFT2 automatic defrost unit thaws the bottled cells. STEMCELL Technologies recommends thawing T cells into T-25 square vials and allowing to stand for two days. Use 5 mL of maximum perfusion of fresh complete medium. This protocol was followed for the first two days of T cell culture, and then the cells were transferred to an Evolve bag for further culture. To determine if it is necessary to perform a static culture step to achieve optimal cell growth and survival, we evaluated another method of directly thawing the bottled cells into a 120 mL Evolve cell culture bag. An Evolve bag of 80 mL working volume was used in this part of the study. Both protocols were sampled and calculated daily using the Vi-CELL Xr Cell Viability Analyzer to monitor cell growth and survival. In addition, Life Technologies' EVOS FL Cell Imaging System was used to inspect cultures in 120 mL Evolve bags. Figure 2 shows human peripheral blood whole T cells taken at 10X magnification.

Figure 2: Growth of T cells in Evolve 120 mL cell culture bags taken at 10X magnification using Life Technologies' EVOS FL cell imaging system
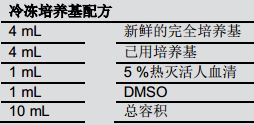
Creating a cryopreserved T cell bank and a frozen medium formulation <br> Creating a cell bank is very important for this study because the purchase of multiple bottles of primary human T cells can be cost prohibitive for extensive research. Once the optimal conditions for T cell expansion are found, a pack of the highest density and most viable Evolve bags is re-frozen back into the cell bank. The formulation of the cryopreserved medium is shown in Table 2. First, 5% of heat-inactivated human serum was added to the cells of the used medium to increase the total serum concentration to 10%. Then, fresh medium was injected into the mixture. Do not use a centrifuge to perform centrifugation on cells, such as standard refreezing, but add DMSO directly to a mixture of cells and medium. Then, dispense it into a 1 mL cryopreservation tube. Subsequently, the cryopreservation tube was placed in a BioCision CoolCell and placed in a New Brunswick Innova U360 ULT refrigerator set at -80 °C overnight. After 24 hours, it was transferred to liquid nitrogen for long-term storage.
Optimizing the culture conditions of the Evolve breathable bag <br> After the thawing method is optimized, the Evolve bag is further evaluated for the desired cell growth and expansion capacity. T cells were thawed directly into Evolve bags with a maximum perfusion of 120 mL, a working volume of 30 mL, and a density of 0.5 x 106 cells/mL. This density level was maintained by adding fresh complete medium until the working volume reached 100 mL. When the bag reaches a working volume of 100 mL, 50% volume replacement can be achieved by removing 50 mL of medium and replacing it with fresh complete medium to maintain a density level of 0.5 x 106 cells/mL.
Comparison of T cell culture in shake flasks and breathable bags <br> In addition, the New Brunswick S41i Co2 constant temperature shaker was used to culture and breathable bags with baffled and unshielded disposable shake flasks at different oscillation speeds. The training program was compared. Each shake flask has a seeding density of 0.5 x 106 cells/mL, utilizing a 20% working volume of a 250 mL shake flask. As a control, T cells were also grown in a 120 mL Evolve cell culture bag with a working volume of 100 mL using the above standard optimal settings and seeding densities.
Use different venting bags to assess the possibility of scale-up <br> After optimizing the 120 mL Evolve bag T cell culture, try to expand the scale, starting with the 500 mL Evolve bag. The situation is consistent with the above.
We also studied the culture effect of a similarly sized breathable bag from another manufacturer. In addition to the large Evolve bags, we also used the Lampire Biological Laboratories OMNI C3 250 mL cell culture bag. Although the Evolve bag has a maximum perfusion volume of 500 mL, we only used a 200 mL working volume to compare it to a Lampire bag with a relatively small working volume.
Results and discussion
Optimizing T cell thawing methods and initial culture <br> We have two thawing methods for T cells, one that adheres to the manufacturer's recommended resting culture hold time and the other does not (see Materials and Methods). Both media were sampled daily and cell growth and viability were monitored. After the initial thawing period, it was further cultured in a cell culture bag, the cell density was maintained at a level of 0.5 x 106 cells/mL, and fresh medium was added to achieve a maximum working volume of 100 mL. Continue to use the method of direct thawing into the bag. Compared with the suspension thawing method, the method is not only more convenient, but also the peak cell density exceeds the former. The data is shown in Figure 3.
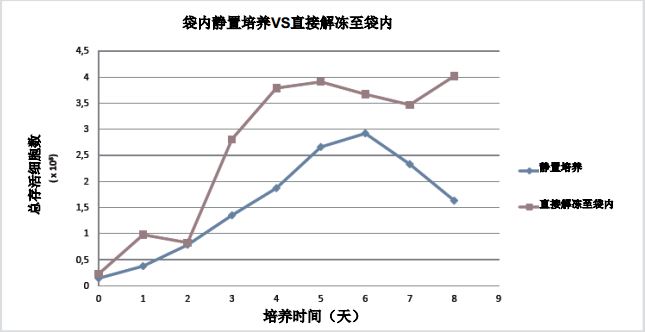
Figure 3: Static thawing method and direct thawing to bag comparison (after thawing, all used for 8 days of bag culture)
Establishing T cell cryopreservation libraries <br> Many manufacturers do not guarantee that cells will survive after cryopreservation. However, we have demonstrated through our own processing techniques and media formulations that cells can be stored for future use (Figure 4).
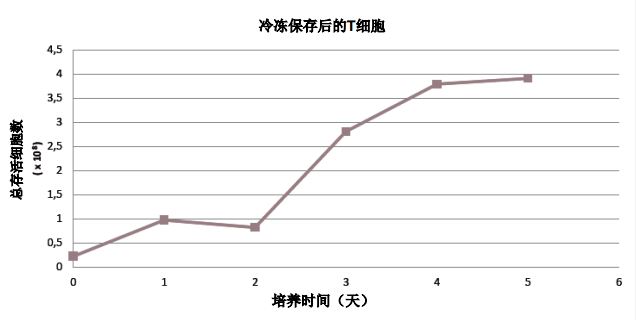
Figure 4: T cells were removed from the cryopreserved cell bank and thawed, cultured in a ventilated bag, and monitored for 5 days.
Comparing T cell culture in shake flasks and breathable bags <br> The cell growth density in the shake flask culture does not reach the cell density level of the ventilated bag culture at the same time. As shown in Figure 5. Since the working volume of the shake flask and the cell culture bag are different, Figure 5 shows the total number of cells obtained with a 50 mL perfusion amount for comparison. A large number of cell pellets were also observed in shake flask cultures at 95 and 100 rpm. No cell pellets were observed in the 70 rpm shake flask and vented bag cultures. In view of the poor performance of shake flask culture, we abandoned the shake flask method, focused on breathable bag culture, and further explored the possibility of scale-up by using larger vented bags.
Use different venting bags to assess the possibility of scale-up <br> To determine if the Evolve bag is an ideal way to expand the size of the bioreactor to inoculate T cells, this study tested a cell culture bag from another manufacturer. Each bag was sampled daily for 7 consecutive days. The results are shown in Figure 6. The density of the Lampire bag of 200 mL working volume is greater than that of the larger size Evolve bag. Although the Evolve bag has a peak density of less than 120 mL, the total cell count of the Lampire bag is 2.5 times higher than that of the large Evolve bag (data not shown). We also tested larger venting bags from 1 L to 5 L, but failed to achieve T cell expansion in the initial evaluation.
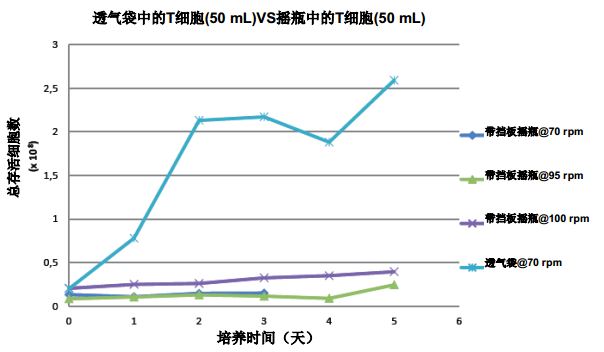
Figure 5: Effect of T cells cultured in plastic shake flasks at different shaking speeds compared to vented bags 
Figure 6: Performance of larger Evolve breathable bags and Lampire breathable bags in human T cell expansion
in conclusion
Through this study, cell banks were successfully established for further experiments. In Evolve breathable bags, T cells expanded significantly. With a 120 mL Evolve breathable bag, the New Brunswick S41i CO2 shaker oscillates a total of 10 Evolve bags. If 10 Evolve bags are filled with 100 mL and the density is 3.91 x 108 cells/bag (Figure 3), then 1 L of liquid can reach a total of 3.91 x 109 T cells. At this density, the BioBLU 3c disposable can inoculate approximately 255 mL of cells at a maximum working volume of 3.75 L. Using a single batch of T cells expanded on a S41i constant temperature shaker, 1 L cells can be seeded with 4 BioBLU 3c disposable cans up to a maximum working volume (3.75 L).
A preliminary study of the Lampire breathable bag also shows the potential to scale up. The Lampire bag has a maximum working volume of 250 mL. In this study, the working volume was 200 mL. The S41i Co2 constant temperature shaker 2 constant temperature shaker oscillating platform can accommodate up to 6 packs of Lampire bags, which can expand the total cell capacity to 1.2 L.
This study demonstrates that a breathable bag with a S41i Co2 constant temperature shaker enables rapid initial expansion of T cells, which is capable of producing sufficient cells to inoculate multiple benchtop bioreactors.
references
[1] Khandaker Siddiquee and Ma Sha. Microcarrier-Based Expansion of Adipose-Derived Mesenchymal Stem Cells in Shake Flasks. Bioprocessing Journal, Volume 12, Issue 4, Winter 2013.
Infusion set,injection products,iv set,iv infusion set,i.v cannula
2 MEDS TECHONOLOGY CO.,LTD , https://www.2-meds.com