Most eye drops are water-based and, in addition, may also contain salts (eg, sodium hydride), a protective agent. Vaseline for the production of ointments and gel polymers for use as lubricants. The heat-sensitive eye drops can be sterilized by sterilization filtration, or the heat-sensitive components can be separately sterilized and filtered, and then added to the raw materials that have been sterilized.
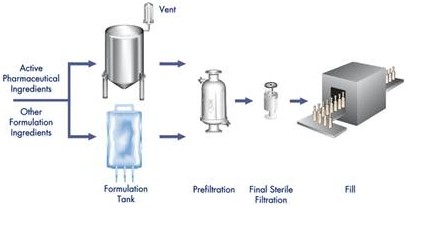
Pre-filtration for separation purposes Reduces particle content and bioburden before sterilization filtration.
The sterile filtration sterilizing filter maintains reliable rejection of microorganisms during prolonged production.
The requirements of the application filter must be able to provide a stable high flow rate and must be very robust for high volume production.
Sterilization filters must be able to maintain reliable retention of microorganisms during prolonged production.
The filter should not adsorb the protective agent in the product.
The filter Polysepll pre-filter is rugged, reliable, able to withstand changes in the production process, and offers high flow rates and reliable rejection for high volume production.
Durapore or a charged ChargedDuropore sterilizing filter has very low adsorption of the protective agent and can also have a 100% sterilization efficiency at high flow rates and high throughput conditions. In many cases, the positively charged ChargedDurapore membrane greatly reduces the adsorption of the protective agent because many of the protective agents are tetraamines with a positive charge. The repulsion between the same charge causes the positively charged Durapore membrane to adsorb very low positively charged protecting agents.
Air filtration, the commonly used filter is Aerovent to ensure sterilization and virus removal.
Clear Sterile Vials are primarily used for mixing different medications or solutions for injection or research applications like HCG, heparin, lidocaine, diabetic medications and morphine for intravenous or syringe injections. Some drugs may need to be diluted or mixed with sterile water or other drugs. Sterile Vials are also used for nuclear medicine, PET-CT, Liquid collection. Clear sterile vials are also used for nuclear medicine, PET-CT, Liquid collection.Clear sterile vials are produced by aluminum caps, non-latex butyl stoppers and clear SCHOTT Neutral Type I glass vials. They are approved by cGMP and FDA with internally sterile.The production process is carried out under strict Class 100 workshop. Finished vials can meet the FDA`s authorised 14-day sterility test.
Clear Sterile Vials
Clear Sterile Empty Vials,Clear Sterile Glass Vials,Clear Sealed Sterile Vials
China Lemon Trading Co.,Ltd , https://www.lemonvial.com